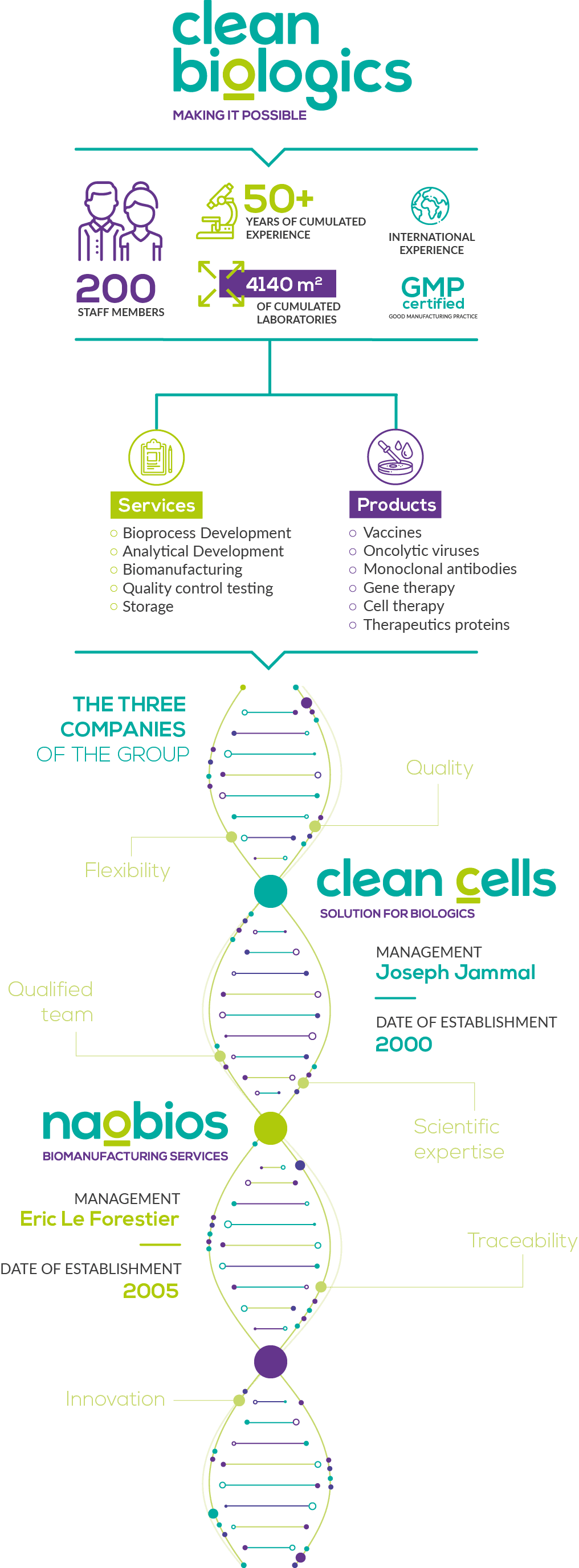
The companies of the groupClean Cells & Naobios:
a complementary services offer !
The cumulated skills and expertise of companies Clean Cells and Naobios are made available through their complementary services offer. The extended list of services are compliant with Good Manufacturing Practices and benefits the global biopharmaceutical industry.
In 2000, three researchers created Clean Cells, a company built around an original identification process for contaminants (mycoplasmas) in cell cultures. The assay received the innovation award from the Aventis – Institut de France foundation, and was designed to ensure that the company’s customers have, indeed, clean cells.
The Vendée-based company has always been managed with a business- and human-oriented vision and has grown consistently for the last 20 years. It offers a full service portfolio including biopharmaceutical quality control testing, cell and virus banking, storage and custom analytical development and validation.
Formerly known as Vivalis, then BE Vaccines, Naobios was founded in 2005 in Saint-Herblain (Nantes area). It was built around a new technology for the production of vaccines.
Naobios also complies with the european GMP framework and has both the capabilities and the expertise to undertake the development of bioprocesses and the manufacture of cell & virus banks, preclinical and clinical lots (viral vaccines and oncolytic viruses) for the pharmaceutical industry.