The quarterly scientific journal

Implementing digital PCR : a quality leap for the quantification of nucleic acids
GMP-certified company Clean Cells has been a longstanding pioneer and advocate for the use of PCR to perform regulatory-based quality control assays (biosafety, identity and purity).
Our expertise has been recognized by multiple institutional and private organizations including regulatory authorities which called upon Clean Cells to improve the framework surrounding animal health products quality evaluation.
Today, our analytical development team is proud to announce the incorporation of innovative digital PCR (dPCR) technology into our service offer to improve precision and sensitivity of quantification assays.
The dPCR method
The innovative nature of this dPCR method particularly lies within the ability to perform absolute quantification of nucleic acids thanks to random partitioning of the mix into thousands of partitions followed by individual analysis of each partition through imaging. Poisson law is used to analyse the statistical distribution of DNA in partitions.
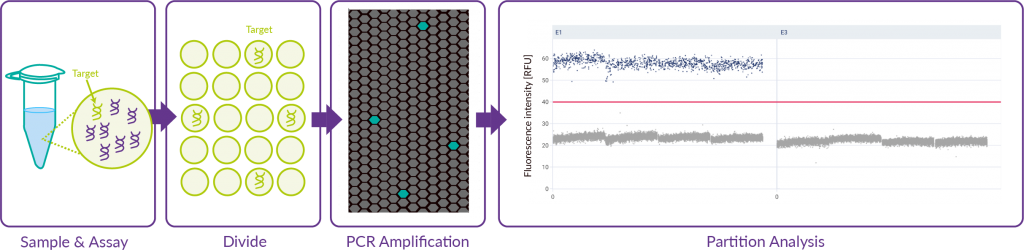
Our equipment sourced from QiAgen allows for additional flexbility of the dPCR technology and the reduction of handling operations and time, thus ensuring increased reliability.
Setting up the method at Clean Cells
Our digital PCR (dPCR) technology may be implemented to perform the following tests and methods:
- Gene Copy Number, Plasmid Copy Number
- Variation analysis: event frequency quantification (translocation/SNP/MNP, etc.)
- Host Cell DNA quantification Quantification (HCD)
- Gene expression
- Titration (vectors, virus seeds, etc.)
Digital PCR may also be used as a complementary method for the folowing assays:
- Virus titration (gene copy number, in combination with TCID50 titration or pfu/mL).
- Cytogenetics (Fluorescence in situ hybridization – FISH)
- Clonality (trangene copy number)
Our team are currently qualifying our Digital PCR methods to implement GMP-ready assays by Q4 2021.
Please reach out to our team to receive our comprehensive portfolio of quality control assays, numbering 200+ validated qPCR tests, for a use in R&D, preclinical or clinical (GMP) setups.