The quarterly scientific journal

Clean Cells, your partner for single cell cloning to cGMP cell banks
As a contract organization specializing in cGMP cell & virus banking and QC testing, Clean Cells knows the importance of qualitative cell line development. We thus implemented Solentim’s VIPS® PRO, a state-of-the-art single cell cloning platform to isolate potent clones and characterize the monoclonality of biotherapeutics producing cell lines, with an increased traceability as part of a regulatory compliant approach.
Read more
Increasing biologics manufacturing capacities
With 23+ years of experience handling hundreds of cell and virus models, Clean Cells has positioned itself as a leader in cell and virus banking, providing complementary characterization and storage services. Our state-of-the-art cGMP facility launched earlier this year now welcomes our biologics manufacturing capacities, providing 8 new clean rooms designed for all types of biopharma projects and equiped to meet production specifications for both adherent and suspension cells.
Read more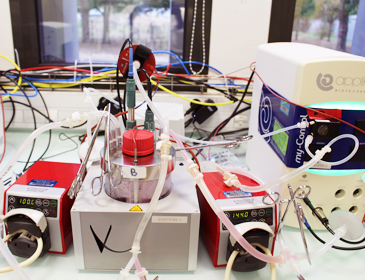
Assessing the scale-X(TM) bioreactor’s biomanufacturing capacities on Vero cells
To further extend their biomanufacturing capabilities and cater to Univercells clients' growing needs, Naobios has recently conducted a comprehensive assessment of the performance of Vero cells cultured within the scale-X(TM) bioreactor provided by Univercells Technologies. This assessment aimed to evaluate the compatibility and efficiency of the Vero cell line in this novel bioreactor system for viral-based bioproduction.
Read more
GMP biomanufacturing in a BSL-3 suite
A GMP biomanufacturing expert, Naobios has worked with numerous viruses requiring specific biocontainment procedures. Our state-of-the-art BSL-3 suite welcomes hazardous microbes used for the generation of virus-based therapeutic products.
Read more