The quarterly scientific journal

Increasing biologics manufacturing capacities
With 23+ years of experience handling hundreds of cell and virus models, Clean Cells has positioned itself as a leader in cell and virus banking, providing complementary characterization and storage services. Our state-of-the-art cGMP facility launched earlier this year now welcomes our biologics manufacturing capacities, providing 8 new clean rooms designed for all types of biopharma projects and equiped to meet production specifications for both adherent and suspension cells.
Read more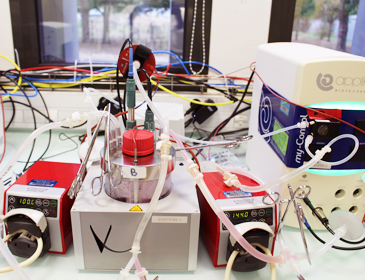
Assessing the scale-X(TM) bioreactor’s biomanufacturing capacities on Vero cells
To further extend their biomanufacturing capabilities and cater to Univercells clients' growing needs, Naobios has recently conducted a comprehensive assessment of the performance of Vero cells cultured within the scale-X(TM) bioreactor provided by Univercells Technologies. This assessment aimed to evaluate the compatibility and efficiency of the Vero cell line in this novel bioreactor system for viral-based bioproduction.
Read more
A new facility to extend biopharma services
Last January, Clean Cells opened its new cGMP-compliant facility, a cutting-edge 5 300 m2 platform which considerably extends biopharma capacities. Additional biologics testing capacity, extended cell and gene therapy services, end-to-end biomanufacturing and storage: Clean Cells’ new site opens a new chapter in the company’s rich history.
Read more
Integrated solution for cell banking and characterization
Clean Cells offers an integrated solution for cell bank generation in GMP and non-GMP-settings together with full characterization services and additional long-term storage options to secure valuable material.
Read more
GMP biomanufacturing in a BSL-3 suite
A GMP biomanufacturing expert, Naobios has worked with numerous viruses requiring specific biocontainment procedures. Our state-of-the-art BSL-3 suite welcomes hazardous microbes used for the generation of virus-based therapeutic products.
Read more